The Chapter Four
Physics
4.1 The reed beds in winter by the shore of Lake Annecy are a nice illustration of how three states of water can coexist naturally on earth – one of many properties that make water a unique substance among the 15 million chemical species we currently know about. They are an equally nice illustration of how the beauty of Lake Annecy can capture the imagination, drawing attention to the miraculous properties of water and the surrounding scenery, and inspiring the onlooker to inquire further into how all this comes to be.
4.2 Dr Servettaz’s ‘L’eau, la vie d’un lac alpin’ is full of such inspiration. Chapter One brings the reader’s attention immediately to the tremendous importance of water thanks to its remarkable qualities and countless external uses, for example as a medium in which the first life arose, as a regulator of the weather, for washing, agriculture, transport, as a generator of energy, a heat exchange, a powerful solvent and much more. His second chapter goes further to show how dependent people are, and all life is, on the countless internal uses of this precious substance: “Made of water, we live in a context created by this fluid”. Drinking, crying, sweating, are merely crude examples of the vast range of its internal functions.
“ Water commands, brings about, organizes all the biochemical exchanges which characterise each type of life-form animal and vegetable” (DRS 27).
4.3 Water flows everywhere throughout Dr Servettaz’s book and therefore also through this website. The arrival of water on the planet is discussed in the section on Cosmology; the role of water in the weather system is discussed in Meterology; water’s role in the local economy is outlined in Water, the life of the economy of Annecy; in the evolution of life, in The first cell; in the formation of life, in Chemistry; in supporting a rapidly expanding global population in Environmental Movement, science, Conclusion; and in recreation, in Tourism. In this section we focus just on the physical (and chemical) properties of water which enable all the uses above.
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
4.4 There are five particular properties of water which are crucial to the life of the lake, and of the planet, and which enable its many hundreds of uses in our daily lives: its abundance on earth 2) evaporation and precipitation, 3) osmosis, 4) capillary action and 5) its ability to dissolve other substances.
4.6 Without the great abundance of water on the planet, if water had been as scarce as gold, then none of what follows would even be possible. Without its ability to evaporate in one place and precipitate in another there would be no rain, and so no rivers and no lakes – all land would be a desert. Without a combination of osmosis, capillary action and evaporation there would be no plants on land, and so no food, no animals and no us. Without its ability to dissolve other substances, such as nutrients, freeing them up to move around and interact inside plants and animals, there would be no life anywhere on the planet.
4.6 But what is behind these five miraculous properties of water? Each separate property appears miraculously useful to our lives, but how do they come about, and is there one simple explanation for them all?
4.6.1 How come there is so much water on earth anyway? Where the water came from which formed Earth’s earliest oceans is discussed in the section on Cosmology. But how abundant is water elsewhere in the universe and where did it come from originally?
4.6.2 Evaporation occurs when individual water molecules escape from the surface of a body of water, and rise up into the air. Water evaporates as steam when it is boiled, but how can water evaporate if it is cool, as for instance on this early morning by the shores of Lake Annecy? How can water, which is heavier than air, rise up into the air to form clouds in apparent defiance of gravity? The picture below shows an aeroplane doing just the same thing at Lake Annecy last summer to fight a forest fire many miles from Annecy. A huge amount of fuel and mechanical energy was involved to move just a few tonnes of water a few kilometres to where it was needed. How can such the insubstantial atmosphere somehow manage to pick up, transport and deposit, just to take one example around 700 tonnes of water a day hundreds of kilometres from the Atlantic across France to drop on the catchment area of Lake Annecy?
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
The hard way to move tonnes of water from one place to another
4.6.3 All living things need water to grow. Animals drink water by sucking, or lapping and swallowing, but plants don't suck, unless you are a bored botany student. So how do plants get the water they need? How do their roots absorb water? If the roots let water in, why don't they let water out at the same time and act as drains instead of pumps for the plant? The answer is a process called osmosis, yet another miraculous property of water, but how does it work?
4.6.4 That water in a glass forms a meniscus, curving up slightly around the edge, is a common sight. If the glass is a tube narrow enough then water will begin to rise up the tube in defiance of gravity. This is called capillary action. But what causes this curious phenomenon, and how can the same phenomenon which enables a little water to rise a few millimetres up a glass tube, also help a giant Sequoia to lift nearly 2000 litres of water through its 100 metre height each day?
4.6.5 Water dissolves many salts and organic molecules such as sugars. Many substances in living organisms, such as proteins, DNA and polysaccharides, are dissolved in water. Water also dissolves many gases, such as oxygen and carbon dioxide. Water’s ability to dissolve many other substances supports not just photosynthesis which kick starts life, but all subsequent processes of biological growth. Water somehow breaks down nutrients into constituent parts, and, just as the ocean provided a constantly moving, supportive environment in which different substances could come together, react in new ways and somehow generate life, so at a microscopic level water enables these different substances to come together, react and form proteins, cytoplasm, DNA and all the reactions in between that drive the daily life of Life. But how and why exactly does water dissolve so many substances?
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
4.7 The answer to all of these questions turns out to be rather elegant and simple.
4.7.1 It is because of the specific atomic structure of a water molecule and the general kinetic theory of matter.
4.7.2 A water molecule is very small – in fact it is the smallest molecule regularly found on earth.
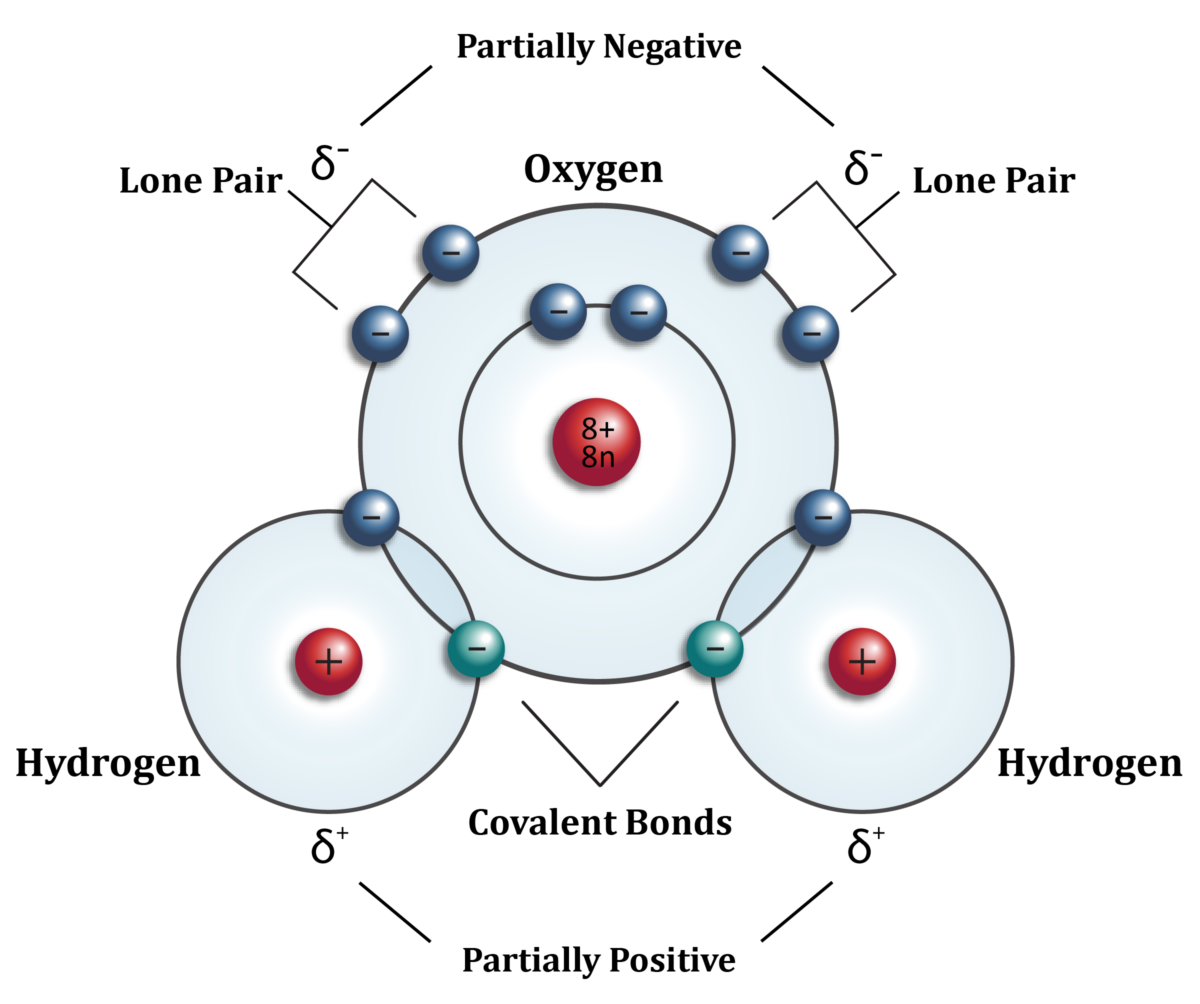
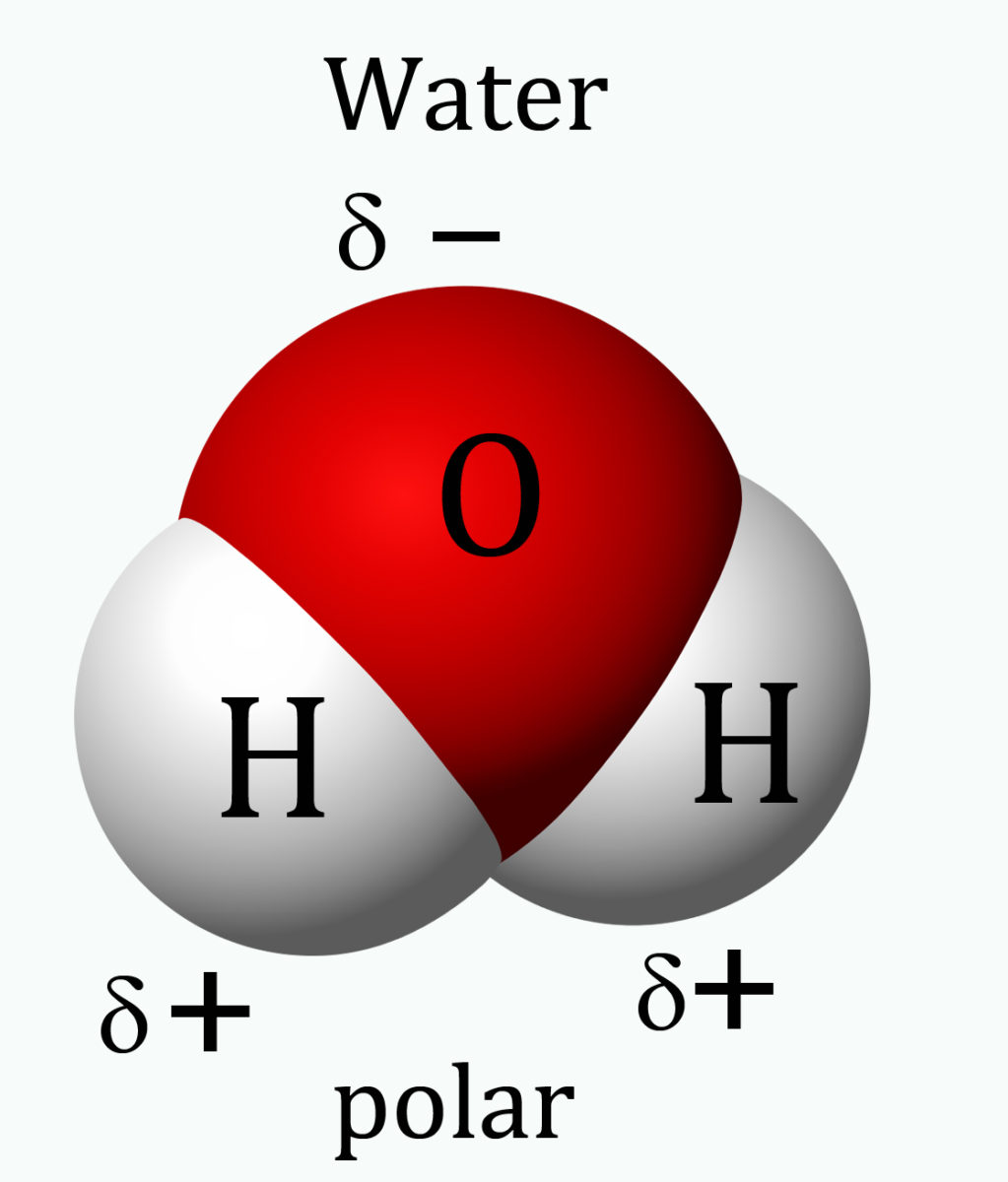
4.7.3 A water molecule is not just very small, its atomic structure is asymmetrical in three dimensions, which gives it polarity – the side where the two hydrogen atoms sit has a slightly positive charge and the opposite side is slightly negatively charged. So the molecule is a ‘di-pole’.
4.7.4 Finally, kinetic theory of matter describes how this molecule, like all molecules, is in constant, random motion, which increases with heat.
4.8 The atomic structure of water looks like this in two dimensions [insert 2 D diagram with explanatory sentence on], but like this in three dimensions [insert 3d diagram with explanatory sentences, to go side by side on the page]. The positively charged hydrogen side of one water molecule is then attracted to, and bonds with, the slightly negatively charged oxygen side of an adjacent water molecule. This is called, not surprisingly, a hydrogen bond. It is these hydrogen bonds which make the water molecules stick together at room temperature and behave as a liquid rather than a gas. In fact, water molecules can bond not just with one other water molecule, but up to four at the same time (each of the two hydrogen atoms of one water molecule bonding with the oxygen sides of one other water molecule, and the oxygen side of the same water molecule binding with hydrogen atoms from two separate water molecules). But these hydrogen bonds are in competition with one other force – kinetic energy which makes the molecules move about in rapid random motion. In fact water is a constant seething mass of molecules constantly being split apart by their kinetic energy and being recaptured by hydrogen bonds. This all happens rather quickly and so is not entirely apparent to the naked eye. In fact bonds between molecules are broken down and reformed about once every femtosecond (1X10-15 seconds or one millionth of one billionth of one second). And so, water molecules exist in a constant tussle between hydrogen bonds that tend to make them stick together and kinetic energy which tends to make them fly apart. The hotter the water the more molecules fly apart, the cooler the more they stick together.
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
Water molecule seen in two dimensions
Water molecule seen in three dimensions
4.9 And this is pretty much all that is needed to explain our five miraculous properties of water as shown below, and in fact all of the innumerable internal and external uses of water for mankind and life on earth. Water is a tiny di-pole in constant rapid motion.
4.9.1 The first elements formed in the universe were, not surprisingly the simplest, hydrogen and helium. These two elements make up respectively around 92% and 7% of the baryonic matter in the universe. Hydrogen is the simplest and lightest in structure, one proton, one neutron and one electron. The other elements i.e. the remaining 1% which comprise pretty much all the world we experience on earth, were produced much later by stellar nucleosynthesis, once clouds of hydrogen and helium had condensed under gravity to produce the first fiery stars. Oxygen was one of the first heavy elements to be generated in this way and is now estimated to be the third most abundant element in the universe. Given the abundance of its components, it is not surprising that water is one of the most abundant compounds naturally occurring in the universe. As NASA observed recently: "The Milky Way and Universe is Awash in Water" April 07, 2015 [include link]
4.9.2 When water evaporates, for instance as we see in the above early morning picture from the surface of Lake Annecy, some molecules of water fly off into the air thanks, as we have seen, to kinetic energy overcoming hydrogen bonds. Now Earth’s atmosphere comprises, amongst other things, around 78% nitrogen and 21% Oxygen, and, as it happens, these two gases occur generally in their diatomic form, i.e. in compounds of two molecules. So the atmosphere comprises mostly molecules with an atomic mass of 28 (N2) and 32 (O2). But the water molecule is tiny, as we have seen, with an atomic mass of just 20 and so has less mass than the air molecules it displaces. These tiny water molecules begin to rise up through the air. However, as the water molecules rise up through cooler and less dense air, they lose kinetic energy and the hydrogen bonds begin to reassert themselves, binding individual molecules together into water droplets. Initially these water droplets are so small that gravity has little effect on them so they float in the air either as early morning mist or as clouds. And as clouds they can be carried many miles by the wind for instance from the Atlantic to Lake Annecy. Only as the density of the water in the clouds increases, or the air temperature decreases, do the individual droplets cohere and grow to such a size that they precipitate and fall as rain.
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
4.9.3 The random movement of tiny water molecules caused by kinetic energy also leads to the phenomenon of osmosis. For instance if you have a container divided by a semi-permeable membrane with pure water on one side and a solution on the other side, then water will begin to migrate from the pure water side to the solution side. This ‘miraculous’ phenomenon is caused by water molecules being tiny and their kinetic energy. Because water molecules are tinier than any other molecule it is possible to create a membrane with holes small enough that allow water molecules to pass but nothing bigger. So kinetic energy will result in water molecules passing both ways entirely at random through the small holes in such as semi-permeable membrane. But here’s the trick. There are relatively more water molecules on the side with pure water than on the side containing the solution – by definition – since a portion of the molecules on the side of the solution are not water. So relatively more molecules with pass from pure water to solution, than vice-versa. And so we see water flow of its own accord across the membrane. The rate of flow is determined by the difference in strength of solution on either side of the membrane, because this is what determines the relative numbers of water molecules randomly jumping across the membrane. Expressions such a osmotic pressure, root pressure etc are simply ways of expressing this mathematical relationship between the numbers of water molecules either side of the membrane.
4.9.4 As described above, up to four hydrogen bonds bind water molecules to each other to give water its cohesive quality, making it stick to itself, as seen in droplets of water or in surface tension on a lake. But these same hydrogen bonds attract water molecules to other substances which happen to be negatively charged. This gives water its adhesive property, when it tends to stick to other things such as glass. When water is placed in a glass test tube its adhesive properties make the water stick to the sides of the glass forming a meniscus. If the glass tube is narrow enough the adhesive and cohesive properties begin to work together – adhesion pulls one water molecule up the side of the tube and cohesion drags the next molecule along with it, and so a column of water rises up the tube in defiance of gravity. This action is called capillary action. This property has caught the imagination of some notable people. Its first recorded observation was by Leonardo da Vinci and Albert Einstein's first paper, which was submitted to Annalen der Physik in 1900, was on capillarity.
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
Giant Sequoia
4.9.4.1 And so we have three entirely different properties of water, evaporation, osmosis and capillary action, working in different ways but driven by the same fundamental atomic structure. Now Nature comes along, in the form of the earliest terrestrial plants, with one of its most brilliant engineering inventions. It devises a mechanism to harness all three properties and so draw water up through the length of the plant in defiance of gravity. The specific invention is a system of two sets of veins called xylem and phloem. These connect the roots of a plant, through its stem all the way up to its leaves. At the roots osmosis for the one way process of absorbing nutrient rich water from the soil, but not letting it leak back out. Next capillary action takes over as water begins to move up the narrow veins of the plant. And the whole upward motion is drive by evaporation of water from the leaves which tends to create a vacuum at the top of the continuous column of water in the plant’s veins, which draws the whole column up from the earth in defiance of gravity. In this way a giant sequoia can transport up to 2000 litres of water a day up through the 100 metres of its trunk. Without the three properties of water combined with the astonishing invention of xylem and phloem, there would be no plants on land, and so no food, no animals and no us.
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
4.9.5 Water can dissolve a wide range of substances thanks to its small size and polarity, but it does this in very different ways depending on the nature of the bonds within the substance in question.
4.9.5.1.1 These bonds are determined by the relative strength of the electronegativity of the elements comprising the substance, which in turn is related to their atomic structures.
4.9.5.1.2 Electronegativity is the tendency of an atom to attract electrons towards itself.
4.9.5.1.3 The strength of electronegativity increases as the number of electrons increases in its outer shell its outer shell increases and decreases the further this outer shell is from the nucleus. When the outer shell is complete the element has zero electronegativity and is entirely non reactive, such as with the noble gases. [Footnote - Linus Pauling devised a scale to measure these – see attached – from Hydrogen with a strength of 2.2 up to Fluorine with a strength of 3.9 all the way down to Caesium with a strength 0.7. Pair with periodic table]
4.9.5.2 If the difference in electronegativity between the atoms in the molecule is less than 0.5, then the bond will be non-polar covalent. A covalent bond is when to atoms share electrons in their outer shell. Non-polar means that, unlike water, the three dimensional structure of the resulting molecule is more or less symmetrical and does have one side with a negative charge and the opposite with a positive charge. The molecular structure is more or less symmetrical because the difference in relative electronegativity is low and so neither atom exerts undue influence on the shape of the resulting molecule.
4.9.5.2.1 If the difference is between 0.5 and 2.0 then the bond will be polar covalent – similar to what we have already seen with water.
4.9.5.2.2 If the difference is greater than 2.0, then the bond will be ionic. An example of an ionic bond is sodium chloride. The sodium atom has one electron in its outer shell and so is very weakly electronegative (0.93 according to Paulin). The chlorine atom lacks just one electron in its outer shell and so is strongly electronegative (3.16). The difference between them is (3.16-0.93) = > 2 and so an ionic bond. This is when the chlorine atom grabs the sodium’s electron for itself to complete its outer shell, leaving sodium also with a complete outer shell. Because the chlorine atom now has an extra electron it becomes a negatively charged version of the atom, which is called a cation. Similarly, because the sodium atom has lost an electron it becomes a positively charged version of the atom, which is called an anion. Since positive charges attract negative charges the cation binds with the anion – and this is called an ionic bond.
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources
4.9.5.3 If substance has non-polar covalent bonding then water cannot dissolve it.
4.9.5.3.1 If a substance is a polar covalent compound such as sugar, then water’s hydrogen bonds get in between the molecules in the compound and overcome the existing bonds between the sugar molecules. Like Francis Drake’s fleet amongst the Spanish Armada. This separates them apart, breaking down the original block of sugar into its individual molecules, but without breaking up the individual molecules, to produce a solution of individual sugar molecules.
4.9.5.3.2 If a substance has an ionic bond, water’s hydrogen bonds will break apart the substance into its individual atoms, and these atoms will be the orginal anions and cations which produced the original ionic bond. Anions and cations of individual elements are particularly reactive and ready for further reactions in due course.
4.9.5.4 And so water has the ability to dissolve a large range of ionic bonded salts into their individual anions and cations, and a large range of polar-covalently bonded substances into their individual molecules, and convey them both at a microscopically small level to the individual cells of plants and animals. At the same time water does not dissolve the cell membranes themselves, because the structural components of cell membranes are made up of lipids which are non-polar compounds. And so plants and animals have all the nutrients they need dissolved to a digestible size, delivered deep inside them to their individual cells, whilst the cell membranes themselves remain undissolved and intact .
4.9.6 How clever is that?
4.9.7 The word miraculous originated to describe the sense of awe and astonishment at witnessing the suspension of the laws of nature by an act of God, for instance by raising people from the dead. It is used throughout this website with a slightly different meaning; namely the sense of awe and astonishment at understanding the operation of the laws of nature, for instance in generating the conditions for life. How the simple atomic structure of water can lead to properties so various and astonishingly useful to life, was, for Dr Servettaz at least, nothing short of miraculous.
4.9.8 If there was one thing which drove Dr Servettaz on his long mission to preserve the quality of water of Lake Annecy it was out of sheer respect for the astonishing and wonderful ways in which water supports life all around us.
Limnology of Lake Annecy
Introduction
1 : Useful charts for reference
2 : Limnology before our Story
Setting the stage – physical sciences
3 : Cosmology
4 : Physics
5 : Chemistry
6 : Geology
7 : Meteorology
Biology 1 - Evolution of life in water:
8 : First life – Prokaryotes
9 : Eukaryota - Algae
10 : Multicellular life - Zooplankton
11 : Fish
Biology 2 - Evolution of life on land:
12 : Plants
13 : Insects
14 : Reptiles & Birds
15 : Mammals
Biology 3 - Intimate life of the Lake:
16 : Cyanobacteria
17 : Algae – Diatoms
18 : Zooplankton - Rotifers, Crustacea
19 : Fish
20 : Plants
21 : Insects
22 : Reptiles & Birds
23 : Mammals
Biology 4 - The Drama:
24 : Eutrophication & safeguarding lakes
25 : INRA Annual Report 2012
26 : Limnology since our Story
27 : Current state of freshwater resources